Neutrophils and monocytes are innate immune cells that are critical for host defense but also contribute to tissue injury associated with inflammatory diseases. An inflammatory response relies on the recruitment of these cells, which occurs through their interaction with the activated endothelium. This is followed by the induction of cellular processes that allow neutrophils and monocytes to engulf targets, release degradative proteinases and reactive oxygen species, and generate cytokines and immunomodulatory molecules that shape the immune response. Both neutrophils and monocytes display plasticity, with the ability to modify their phenotype depending on the local environment, which, in turn, negatively or positively influences inflammatory outcomes. Dr. Mayadas’ laboratory is interested in understanding the mechanisms of neutrophil and monocyte-mediated inflammation in the context of renal autoimmune disorders, cancer and infectious diseases. Her group focuses on:
- determining the molecular basis of neutrophil recruitment, activation and function,
- further understanding the recently appreciated role of highly immunogenic neutrophil-derived antigen presenting cells (nAPCs) in regulating the adaptive immune response, which challenges the traditional view of neutrophils contributing only to early phases of inflammation,
- delineating the mechanisms driving the generation of nAPCs,
- elucidating pathways of monocyte recruitment and phenotypic changes responsible for inflammation induced renal injury.
Her group uses a broad range of molecular, cellular and imaging approaches on isolated primary leukocytes and endothelial cells, genetically engineered mice subjected to murine models of disease and human samples for their studies.
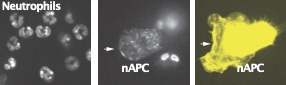
NEUTROPHIL CONVERSION TO nAPCs
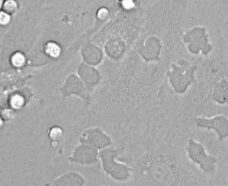
MONOCYTE ADHESION TO ENDOTHELIUM
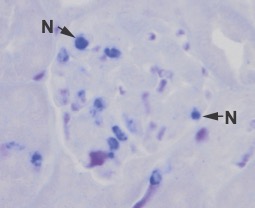
NEUTROPHILS IN THE INFLAMMED GLOMERULUS
GLOMERULONEPHRITIS
*
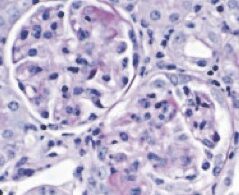
A major interest of the laboratory is to elucidate the contribution of adhesion receptors and associated signaling pathways in regulating the activation and function of neutrophils that is facilitated by knock-out and humanized transgenic animals developed by her group. Her lab has established low affinity human neutrophil FcγRs (receptors for IgG antibody-antigen immune complexes) and the integrin, Mac-1 (CD11b/CD18, complement receptor 3) as primary mediators of neutrophil recruitment and associated organ damage in IgG-mediated glomerulonephritis, a leading cause of renal failure worldwide. Moreover, they have identified regulators of FcgR affinity and function. Recent work has demonstrated that FcγR engagement of neutrophils with immune complexes or an anti-FcγR-antigen conjugate converts them into highly immunogenic antigen presenting cells (nAPC), a finding that may contribute to the pathogenesis of immune-complex-mediated autoimmune diseases but may also be harnessed for cancer immunotherapy. Her group went on to exploit these nAPCs to identify protective, heterologous T cell immunity in COVID-19 induced by trivalent Measles-Mumps-Rubella (MMR) and Tetanus-Diphtheria-Pertussis (Tdap) vaccines. These recent discoveries have led to a major effort in the lab to understand the role of nAPCs in autoimmune disorders, cancer and infectious diseases.
Monocytes play a major role in renal inflammation. The lab has elucidated a role for renal TNF receptor 2 and downstream autocrine IFNβ signaling in monocyte recruitment and subsequent renal damage in IgG-mediated crescentic glomerulonephritis. Recent work in progress has extended these findings and elucidated pathways important for monocyte conversion to inflammatory monocytes/macrophages in the kidney.